Non-Volatile Residue (NVR)Testing System & Solutions
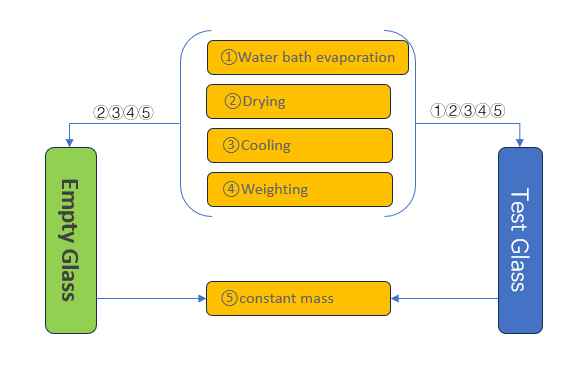
NVR determination involves evaporating the organic solvent, then measuring the residue gravimetrically using a sensitive balance and carefully-tared weighing vessels. Labthink Non-volatile residue (NVR) Testing System is featured as
- Fully-closed, zero leakage water bath avoids harmful gas overflow.
- High precision electronic scale with a repeatability up to 0.05mg.
- Water bath evaporation, drying, cooling and weighing at room temperature are completed automatically.
- Highly efficient reagent collection
Our Labthink Non-volatile residue (NVR) Testing System provides an opportunity to:
- Determination of various chemical reagent residues after evaporation
- Total migration testing of food contacting materials
- Determination of non-volatile matters in purified water
- Determination of non-volatile matters of various pharmaceutical Packaging materials
View the product details below to discover more applications of our Non-volatile residue (NVR) Testing System or ask us for more.
C840H Integrated Evaporation Residue Testing System
C840H Integrated Evaporation Residue Testing System is designed and produced based on the principle of gravimetric method measurement and testing standards for plastic packaging and chemical reagents, etc. It is suitable for the determinations of evaporation residue of food or pharmaceutical packaging, total migration of food contact or pharmaceutical contact materials or products, and evaporation residue of chemical reagents and purified water.

