Validated instruments are the foundation of pharmaceutical security, supporting reliable, data-driven decisions on packaging drugs and pharmaceuticals throughout their shelf life and the rigors of global distribution.
A single inaccurate test result can lead to catastrophic consequences, ranging from compromised patient safety to costly product recalls. So, how to build a validation plan for pharmaceutical packaging test instruments? This guide will give you some hints.
Key Takeaways
- Regulatory Alignment: Instrument validation is a mandatory component of Current Good Manufacturing Practice(cGMP) and process validation.
- The Lifecycle Approach: Effective validation follows a structured DQ/IQ/OQ/PQ framework.
- Risk-Based Strategy: High-impact tests,such as barrier and integrity analysis, require deeper validation scrutiny.
- Data Integrity: Modern validation must address 21 CFR Part 11 compliance for electronic records and audit trails.
Why Instrument Validation Matters in Pharmaceutical Packaging
In the context of life sciences, packaging is an integral part of the drug-delivery system. If a test instrument provides inaccurate data, it can hide critical defects, such as micro-leaks or, conversely, create " false rejections " that lead to significant material waste and supply chain delays.
Regulatory bodies, including the FDA and EMA, view packaging validation as an extension of the overall manufacturing process.
Under 21CFR Part 211, manufacturers must demonstrate that laboratory facilitiesand equipment are suitable for their intended use. This aligns with the "lifecycle validation " concept, where an instrument’s performance is monitored and maintained from installation through its entire operational lifeto ensure that the drug product remains safe and effective for the patient.
Quality and Regulatory Framework for Packaging Validation
The regulatory architecture for pharmaceutical packaging ensures that every testing process is controlled, documented, and reproducible. To meet the expectations of agencies like the FDA or EMA, laboratory equipment must be integrated into a formal Quality Management System (QMS).
Core Regulatory Expectations
- User Requirement Specifications (URS):You must document exactly what the instrument needs to achieve before it is purchased. This includes defining the necessary measurement range, sensitivity levels, and environmental tolerances for your specific drug products.
- Equipment Qualification: Regulators require a tiered approach to validation, typically divided into Design (DQ),Installation (IQ), Operational (OQ), and Performance (PQ) qualifications. Each stage acts as a mandatory gate for compliance.
- Standardized Methodologies: High-end testing must follow recognized international standards such as ISO 11607, ISO 17025,ASTM F1929 for dye penetration, or ASTM F2097 for medical packaging. These provide the scientific basis for your testprotocols.
- Acceptance Criteria: Every validation plan must include predetermined " pass or fail " limits. These benchmarks ensure the instrument consistently meets the performance standards required for patient safety.
Integration of Laboratory Instruments
Testing instruments are the hardware foundation of the validation framework. Whether you are measuring gas permeability or seal strength, these devices must be included in the site’s Master Validation Plan.
- Integrity and Barrier Testing: Systems used for Oxygen Transmission Rate (OTR) and Water Vapor Transmission Rate(WVTR) must prove they can detect minute variations that could affect shelf life.
- Mechanical Performance: Instruments for tensile, burst, and peel testing must demonstrate repeatable results to ensure the package survives the physical stresses of the supply chain.
- Data Integrity and Security: Modern frame works place heavy emphasis on electronic records. Systems must comply with21 CFR Part 11, featuring secure user logins, encrypted data storage, and comprehensive audit trails that record every modification or test run.
We have concluded the structure approach to help you establish a record of quality that satisfies even the most rigorous regulatory audits.
WhichPharmaceutical Packaging Test Instruments to Include?
Selecting the test instruments requires risk-based thinking where the depth of validation is determined by how much a specific test impacts the stability of the drug and the safety of the patient.
Barrier Property Systems
Instruments measure the Oxygen Transmission Rate and Water Vapor Transmission Rate of materials. In pharmaceutical applications, they are used to test high barrier films, blister packs, and bottles.
The precise sensor technology is used to ensure that moisture-sensitive or oxygen-sensitive formulations do not degrade over their intended shelf life.
→ Get to know
Labthink Water Vapor Permeability Testers
Labthink Oxygen Transmission Rate Tester
Package Integrity and Leak Testers
These systems utilize deterministic methods such as vacuumdecay or pressure decay to detect microscopic breaches in the package seal. These are essential for sterile drug products where even a minor leak could lead to microbial contamination or loss of efficacy.
→ Get to know
Labthink Package Seal Integrity Testing / Package Leak Testers
Mechanicaland Physical Performance Testers
This category includes equipment for tensile testing, peel strength analysis, and burst resistance.
For pharmaceutical applications, these instruments verify that the seal strength is sufficient to maintain a sterile barrier while still allowing for easy opening by a healthcare provider or patient.
→ Get to know
Labthink tensile testing machines
Torque and Closure Testers
These instruments measure the opening and closing force for bottle caps and child resistant closures. They ensure that the packaging remains securely sealed during distribution while remaining functional for the end user.
→ Get to know
Labthink Bottle Cap Torque Tester
Environmental Conditioning Chambers
These chambers expose packaging sample to controlled temperature and humidity levels before testing. This is acritical step for stability studies and for simulating the various climates a product may encounter in the global supply chain.
→ Get to know
Environmental Requirements for Material Testing of Package
Risk-BasedApproach to Validation
- Impact on Product Quality: Instruments testing primary packaging that directly contacts the drug require a higher level of validation depth compared to secondary packaging testers.
- Method Complexity: Automated systems with complex software algorithms for data analysis require more rigorous operational qualification to ensure the integrity of the results.
- Frequency of Use: Equipment used for high volume batch release testing should undergo more frequent performance verification to ensure consistent accuracy over time.
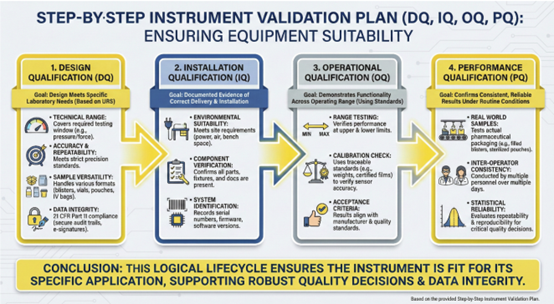
Step-by-Step Instrument Validation Plan covering DQ, IQ, OQ, and PQ
The validation process follows a logical lifecycle designed to ensure that laboratory equipment is suitable for its specific application. This structured approach moves from the initial planning stages to real-world performance testing in the laboratory environment.
DesignQualification and User Requirements
Design Qualification is the first step in the validation process. It involves a documented review to ensure that the instrument design meets the specific needs of the pharmaceutical laboratory. This stage is built upon the User Requirement Specification which defines the technical boundaries of the equipment.
- Technical Range: The instrument must cover the required testing window such as the specific pressure range for leak detection or the force range for seal strength testing.
- Accuracy and Repeatability: The equipment must meet strict precision standards to ensure that test results are reliable across different batches.
- Sample Versatility: The hardware shouldbe capable of testing a variety of pharmaceutical formats including blisters, vials, pouches, and IV bags.
- Data Integrity: The software must support compliance with 21 CFR Part 11 by providing secure audit trails andelectronic signatures.
InstallationQualification
Installation Qualification provides documented evidence that the instrument has been delivered and installed correctly. This stage ensures that the laboratory environment is prepared to support the high precision requirements of the equipment.
- Environmental Suitability: Verification that the installation site meets the necessary requirements for power, compressed air, and stable bench space.
- Component Verification: Confirmation that all parts, specialized fixtures, and technical documentation are present and undamaged.
- System Identification: Accurate recording of the instrument serial numbers, firmware versions, and software configurations for the permanent validation record.
OperationalQualification
Operational Qualification focuses on demonstrating that the instrument functions as intended across its entire operating range. During this phase, the laboratory tests the equipment using reference materials or calibrated standards rather than actual drug products.
- Range Testing: Verification that the instrument performs accurately at its upper and lower limits as defined in the user requirements.
- Calibration Check: Using traceable standards such as certified reference films for barrier testing or calibrated weights for mechanical testing to verify sensor accuracy.
- Acceptance Criteria: The results must align with predefined limits established by the manufacturer and the laboratory quality standards.
Performance Qualification
Performance Qualification is the final stage where the instrument is tested under actual routine operating conditions. This phase confirms that the system delivers consistent and reliable results when used with real pharmaceutical packaging types.
- Real World Samples: Testing actual packaging components such as filled blister packs, sterilized pouches, or capped vials.
- Inter Operator Consistency: Conducting tests with different trained laboratory personnel over multiple days to ensure the process is robust.
- Statistical Reliability: Evaluating the repeatability and reproducibility of the data to confirm that the instrumentcan support critical quality decisions.
TL;DR: 1-Minute to Know the Right Testing Infrastructure for Your Pharmaceutical Packaging
To maximize efficiency and ensure long-term compliance, laboratories should consider several practical factors when choosing and qualifying their testing equipment.
Alignment with Global Standards
Select instruments that are specifically engineered to support relevant pharmaceutical standards such as those from ASTM, ISO, and USP. Equipment designed with these methodologies in mind simplifies the transition from installation to routine testing.
Focus on Data Integrity
In a regulated environment, the software is as important as the hardware. Prioritize systems that offer built in support for 21 CFR Part 11. This includes secure user management, encrypted data storage, and automated audit trails that cannot be modified or deleted.
Hardware Stability and Precision
High-end pharmaceutical testing requires extreme sensitivity, especially for barrier and leak analysis. Look for instruments with proven sensor stability and repeatable calibration procedures to minimize the risk of OQor PQ failures.
Vendor Support and Documentation
The validation process is much faster when the equipment manufacturer provides comprehensive support. Reputable vendors offer prewritten IQ, OQ, and PQ protocols that are tailored to the specific instrument, which significantly reduces the administrative burden on your quality team.
Training and Ongoing Service
Proper validation requires that operators are fully trained and that the equipment is maintained in a qualified state. Choose a partner that offers professional training and scheduled calibration services to ensure the instrument remains compliant throughout its entire lifecycle.
Contact Labthink for Precise Packaging Testing Instruments & Testing Services
Labthink is a global leader in the design and manufacture of high precision testing instruments for the pharmaceutical industry. We specialize in providing advanced solutions for barrier properties, package integrity, and mechanical strength, all engineered to meet the rigorous demands of international standards.
Our team offers extensive support for the validation process, including detailed documentation and technical services to help your laboratory achieve and maintain compliance.
To explore our full range of pharmaceutical testing solutions and validation support services, please visit our website at Labthink.